

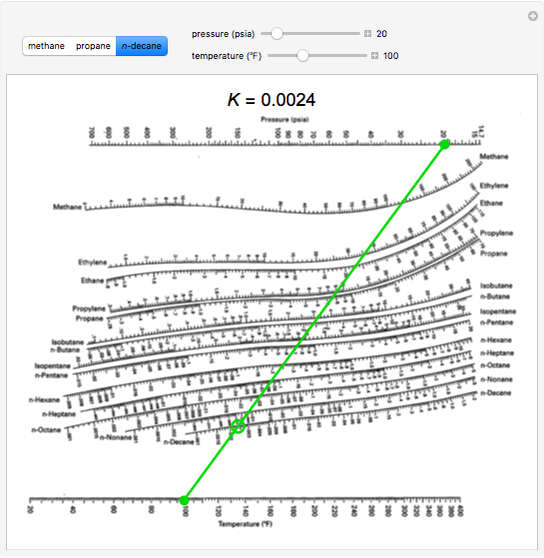
Therefore, scientists and engineers have developed numerous curve fitted expressions for calculation of K-values. In addition, this method ignores the fact that the K-values are composition dependent.Īs mentioned earlier, determination of K-values from charts is inconvenient for computer calculations. At temperatures above the critical point of a component, one must extrapolate the vapor pressure which frequently results in erroneous K-values. This method is simple but it suffers when the temperature of the system is above the critical temperature of one or more of the components in the mixture. Raoult’s law is applicable to low pressure systems (up to about 50 psia or 0.35 MPa) or to systems whose components are very similar such as benzene and toluene. Complex vapor pressure equations such as presented by Wagner, even though more accurate, should be avoided because they can not be used to extrapolate to temperatures beyond the critical temperature of each component. Values of A, B, and C for several compounds are reported in the literature. įor computer use, later in 1958 these K-Value charts were curve fitted to the following equations by academic and industrial experts collaborating through the Natural Gas Association of America. The determination of convergence Pressure is a trial-and-error procedure and can be found elsewhere. In order to use these charts, one should determine the Convergence Pressure first. In more recent publications, the K-values are plotted as a function of pressure on the x-axis with temperature and Convergence Pressure as parameters. This pressure was termed the “Convergence Pressure” of the system and has been used to correlate the effect of composition on K-values, thus permitting generalized K-values to be presented in a moderate number of charts. In each chart the pressure range is from 70 to 7000 kPa (10 to 1000 psia) and the temperature range is from 5 to 260 ✬ (40 to 500 ✯).Įarly high pressure experimental work revealed that, if a hydrocarbon system of fixed overall composition were held at constant temperature and the pressure varied, the K-values of all components converged toward a common value of unity (1.0) at some high pressure. In these charts, K-values for individual components are plotted as a function of temperature on the x-axis with pressure as a parameter. One of the earliest K-value charts for light hydrocarbons is presented in reference. There are several forms of K-value charts.

EoS approach requires use of a digital computer. The widely used approaches are K-value charts, Raoult’s law, the equation of state (EoS) approach (f), activity coefficient approach (?) or combination of EoS and the EoS and ? approaches.
#DEPRIESTER CHART FOR HYDROGEN PLUS#
The components making up the system plus temperature, pressure, composition, and degree of polarity affect the accuracy and applicability, and hence the selection, of an approach. In general K-values are function of the pressure, temperature, and composition of the vapor and liquid phases. This “Tip of the Month” presents a history of many of those graphical methods and numerical techniques. Alternatively, there are several graphical or numerical tools that are used for determination of K-values. Obviously, experimental measurement is the most desirable however, it is expensive and time consuming. Equation (2) is also called “Henry’s law” and K is referred to as Henry’s constant. For the more volatile components the Kvalues are greater than 1.0, whereas for the less volatile components they are less than 1.0.ĭepending on the system under study, any one of several approaches may be used to determine K-values. Ki is called the vapor–liquid equilibrium ratio, or simply the K-value, and represents the ratio of the mole fraction in the vapor, yi, to the mole fraction in the liquid, xi.


 0 kommentar(er)
0 kommentar(er)
